Here're the instructions:
PART A
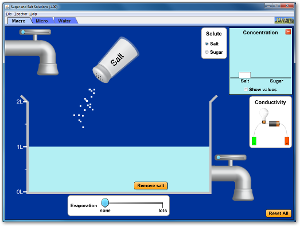
1) Click on the image below to download and run the flash software.
2) Drag the conductivity tester into the beaker of water. Place the negative and positive electrodes into the water, but not touching the bottom of the beaker.
3) Click on the salt shaker and drag it to shake. Does salt dissolve? Does it conduct electricity? Why do you think the solution can/cannot conduct electricity? Record your observations and thoughts in the given table.
4) Repeat the experiment with the sugar shaker.
PART B
1) Click on the 'Micro' tab at the top left hand corner.
2) Shake salt into water. Before the salt lands on the water, pause the animation. What do you think will happen to the salt particles?
3) Resume the animation. Are your predictions right?
4) Repeat the experiment for sugar.
5) Click on the 'Water' tab at the top left hand corner to confirm your predictions.
PART C
1) Watch the video on the heating of salt below. How do you think the colour and state of salt would change over time? Why? Record your observations in the given table.
2) Repeat the experiment for sugar. Discuss your observations and try to think of a suitable reason for it with your friend.
CONCLUSION
By now, you should be familiar with the electrical conductivity and melting points of ionic and covalent compounds.